Ancestral Reconstruction using Discrete Phylogeography
by Walter Xie, Remco Bouckaert, and Alexei Drummond
If you haven’t installed LPhy Studio and LPhyBEAST yet, please refer to the User Manual for their installation. Additionally, this tutorial requires other third-party programs, which are listed below under the section Programs used in this tutorial.
This tutorial guides you through a discrete phylogeography analysis of a H5N1 epidemic in South China. The analysis will use the model developed by Lemey et al., 2009 that implements ancestral reconstruction of discrete states (locations) in a Bayesian statistical framework, and employs the Bayesian stochastic search variable selection (BSSVS) to identify the most plausible description of the phylogeographic diffusion process.
The additional benefit in using this model is that we can summarise the phylogeographic inferences from an analysis, and use a virtual globe software to visualise the spatial and temporal information.
The NEXUS alignment
The data is available in a file named H5N1.nex. If you have installed LPhy Studio, you don’t need to download the data separately. It comes bundled with the studio and can be accessed in the “tutorials/data” subfolder within the installation directory of LPhy Studio.
This alignment is a subset of original dataset Wallace et al., 2007, and it consists of 43 influenza A H5N1 hemagglutinin and neuraminidase gene sequences. These sequences were isolated from a diverse range of hosts over the period from 1996 to 2005 across various sampling locations.
Loading the script “h5n1” to LPhy Studio
To get started, launch LPhy Studio.
To load the script into LPhy Studio, click on the File menu
and hover your mouse over Tutorial scripts.
A child menu will appear, and then select the script h5n1.
The probabilistic graphical model will be visualised on the left panel.
By clicking on the graphical components, you can view their current values
displayed in the right-side panel titled Current.
You can also switch the view to show the script after clicking on the tab Model.
The next tab, Narrative, will display an auto-generated narrative
using natural language to describe the models with citations.
This narrative can serve as the foundation for manuscript method sections.
If you are not familiar with LPhy language, we recommend reading the language features before getting started.
Code
data {
options = {ageDirection="forward", ageRegex="_(\d+)$"};
D = readNexus(file="data/H5N1.nex", options=options);
L = D.nchar();
taxa = D.taxa();
D_trait = extractTrait(taxa=taxa, sep="_", i=2);
K = D_trait.canonicalStateCount();
dim = K*(K-1)/2;
dataType = D_trait.dataType();
}
model {
π ~ Dirichlet(conc=[2.0, 2.0, 2.0, 2.0]);
κ ~ LogNormal(meanlog=1.0, sdlog=1.25);
γ ~ LogNormal(meanlog=0.0, sdlog=2.0);
r ~ DiscretizeGamma(shape=γ, ncat=4, replicates=L);
Θ ~ LogNormal(meanlog=0.0, sdlog=1.0);
ψ ~ Coalescent(taxa=taxa, theta=Θ);
D ~ PhyloCTMC(Q=hky(kappa=κ, freq=π), mu=0.004, siteRates=r, tree=ψ);
π_trait ~ Dirichlet(conc=rep(element=3.0, times=K));
I ~ Bernoulli(minSuccesses=dim-2, p=0.5, replicates=dim);
R_trait ~ Dirichlet(conc=rep(element=1.0, times=dim));
Q_trait = generalTimeReversible(rates=select(x=R_trait, indicator=I), freq=π_trait);
μ_trait ~ LogNormal(meanlog=0, sdlog=1.25);
D_trait ~ PhyloCTMC(L=1, Q=Q_trait, dataType=dataType, mu=μ_trait, tree=ψ);
}
Graphical Model
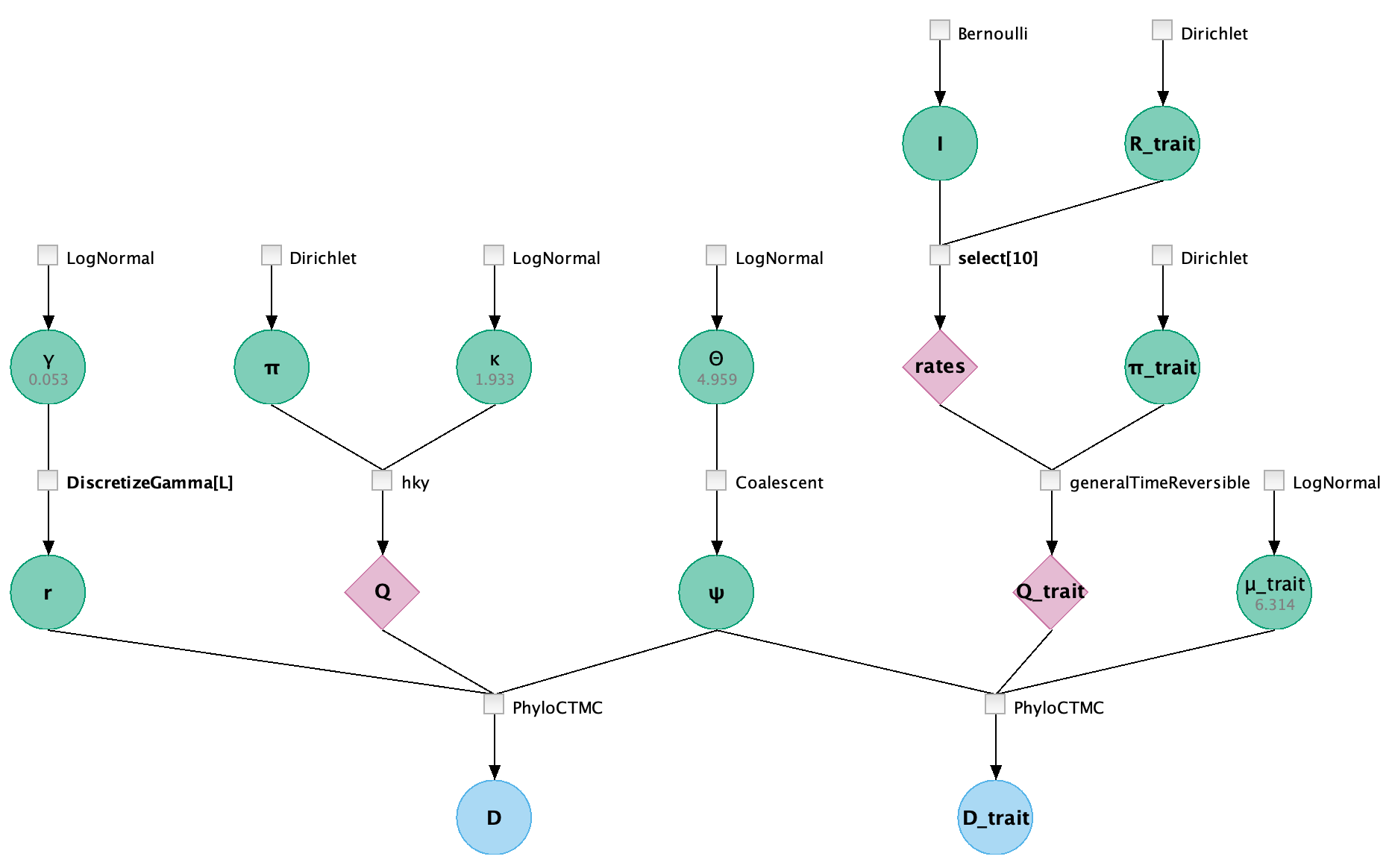
Data block
In the data block, we begin by defining an option to extract the sample times
from the taxa labels using a regular expression _(\d+)$,
and we treat those times as dates (i.e., moving forward in time).
For instance, the taxon “A_chicken_Fujian_1042_2005” will yield the year 2005,
making the age of this tip 0 since 2005 is the latest year among these samples.
Clicking on the orange diamond labeled “taxa”, you can see all taxa along with their ages,
which have been converted from the years extracted from their labels.
If it is not in the probabilistic graphical model, please select the checkbox Show constants
to display the full model.
Next, we read the file “H5N1.nex” and load it into an alignment D,
using the previously defined options.
Following that, we assign the constant L to represent the number of sites in alignment D,
and we retrieve the vector of taxa names from the alignment D.
The line using the function extractTrait extracts the locations from the taxa names
by splitting the string by “_” and taking the 3rd element (where i starts at 0).
This code creates a trait alignment D_trait containing the locations mapped to each taxon.
Then the method canonicalStateCount() counts the number of unique canonical states (locations)
in the trait alignment D_trait and assigns this number to the constatnt K.
This method excluds ambiguous states.
The constant dim used as the dimension for sampling discrete traits is computed from K.
Model block
In the model block, we have mixed two parts.
The first part is modeling evolutionary history and demographic structure
based on a nucleotide alignment,
and the second part is defining how to sample the discrete states (locations)
from the phylogeny shared with the 1st part.
Coalescent based phylogenetic model
The canonical model in the first part of this analysis includes the following parameter distributions:
- Dirichlet prior on,
π, the equilibrium nucleotide frequencies (with 4 dimensions, one for each nucleotide); - LogNormal prior on,
κ, the transition/transversion ratio; - LogNormal prior on,
γ, the shape parameter of discretize Gamma; - Discretized gamma prior on,
r, the vector of site rates with a rate for each site in the alignment; - LogNormal prior on,
Θ, the effective population size; - Coalescent (constant-size) prior on,
ψ, the time-scaled phylogenetic tree which is scaled according to the unit of sampling time associated with taxa. The time unit in this data is years.
Finially, the phylogenetic continuous-time Markov chain distribution, PhyloCTMC,
utilizes the instantaneous rate matrix Q retured from a deteminstic function hky,
the site rates r, and the simulated Coalescent tree ψ as inputs,
along with a fixed value of 0.004 for the mutation rate, to generate the alignment D.
For more details, please read the auto-generated narrative from LPhy Studio.
Geographic Model
The next part is the geographic model. In the discrete phylogeography, the probability of transitioning to a new location through the time is computed by
\[P(t) = e^{\Lambda t}\]where $\Lambda$ is a $ K \times K $ infinitesimal rate matrix, and $K$ is the number of discrete locations (i.e. 5 locations here). Then,
\[\Lambda = \mu S \Pi\]where $\mu$ is an overall rate scalar, $S$ is a $ K \times K $ matrix of relative migration rates, and $\Pi = diag(\pi)$ where $\pi$ is the equilibrium trait frequencies. After the normalization, $\mu$ measures the number of migration events per unit time $t$. The detail is explained in Lemey et al., 2009.
So, assuming migration to be symmetric in this analysis:
- We define a vector variable
R_traitwith the length of $ \frac{K \times (K-1)}{2} $ to store the off-diagonal entries of the unnormalised $S$, which is sampled from a Dirichlet distribution. - A boolean vector
Iwith the same length determines which infinitesimal rates are zero, and it is sampled from the vectorized functionBernoulliusing the keywordreplicates. This along with the deterministic functionselectimplements the Bayesian stochastic search variable selection (BSSVS). - The base frequencies
π_traitare sampled from a Dirichlet distribution. - The deteminstic function
generalTimeReversibletakes relative rates filtered byselect, which selects a value if the indicator is true or returns 0 otherwise, along with base frequencies to produce the general time reversible rate matrixQ_trait. - The migration rate,
μ_trait, is sampled from a LogNormal distribution.
In the end, the 2nd PhyloCTMC takes the instantaneous rate matrix Q_trait,
the data type from the clamped alignment D_trait imported in the data block,
the migration rate μ_trait, the same tree ψ sampled from the 1st part of this model as inputs,
to simulate the location alignment D_trait whose supposes to have only one site.
As you have noticed, there are two D_trait in this script, one is in the data block,
the other is in the model block. This is called data clamping.
The detail can be refered to either the section “2.1.11 Inference and data clamping”
in the LPhy paper
or visit the LPhy language features page.
Producing BEAST XML using LPhyBEAST
BEAST 2 retrieves the data and model specifications from a XML file. One of our goals with LPhy is to make configuring Bayesian phylogenetic analysis as painless, clear and precise as possible. In order to achieve that, we will utilize an additional companion application called LPhyBEAST, which acts as a bridge between the LPhy script and the BEAST 2 xml. It is distributed as a BEAST 2 package, please follow the instruction to install it.
To run LPhyBEAST and produce a BEAST 2 xml, you need to use the terminal
and execute the script lphybeast.
The LPhyBEAST usage can help you get started.
In our h5n1.lphy script, the alignment file is assumed to be located
under the subfolder tutorials/data/.
To generate the XML, navigate to the tutorials folder where LPhy is installed,
and run the following command in your terminal.
After it completes, check the message in the end to find the location of the generated XML file.
For example, on a Mac, after replacing all “x” with the correct version number,
you can execute the following lphybeast command in the terminal to launch LPhyBEAST:
# go to the folder containing lphy script
cd /Applications/lphystudio-1.x.x/tutorials
# run lphybeast
'/Applications/BEAST 2.7.x/bin/lphybeast' h5n1.lphy
If you are not familiar with inputting valid paths in the command line, here is our Tech Help that may help you.
For Windows users, please note that “C:\Program Files” is usually a protected directory.
However, you can copy the “examples” and “tutorials” folders with “data”
into your “Documents” folder and work in that location to avoid any permission issues.
Alternatively, run lphybeast.bat on a Windows terminal like this:
# go to the subfolder containing lphy script
cd "C:\Users\<YourUserName>\Documents\tutorials"
# run lphybeast
"C:\Program Files\BEAST2.7.x\bat\lphybeast.bat" h5n1.lphy
Please note that the single or double quotation marks ensure that the whitespace in the path is treated as valid.
If you want the longer chain length, you can use the -l option to modify the MCMC chain length in the XML.
The default is 1 million.
After LPhyBEAST completes the run, it will print a location information for the BEAST 2 XML at the end of the message.
Running BEAST
After LPhyBEAST generates a BEAST 2 .xml file (e.g., h5n1.xml), we can use BEAST 2 to load it and start the inferential MCMC analysis. BEAST 2 will write its outputs to disk into specified files in the XML, and also output the progress of the analysis and some summaries to the screen.
The h5n1.xml is also provided as an additional resource, in case you need to use it for your analysis.
BEAST v2.7.8, 2002-2025
Bayesian Evolutionary Analysis Sampling Trees
Designed and developed by
Remco Bouckaert, Alexei J. Drummond, Andrew Rambaut & Marc A. Suchard
Centre for Computational Evolution
University of Auckland
r.bouckaert@auckland.ac.nz
alexei@cs.auckland.ac.nz
Institute of Evolutionary Biology
University of Edinburgh
a.rambaut@ed.ac.uk
David Geffen School of Medicine
University of California, Los Angeles
msuchard@ucla.edu
Downloads, Help & Resources:
http://beast2.org/
Source code distributed under the GNU Lesser General Public License:
http://github.com/CompEvol/beast2
BEAST developers:
Alex Alekseyenko, Trevor Bedford, Erik Bloomquist, Joseph Heled,
Sebastian Hoehna, Denise Kuehnert, Philippe Lemey, Wai Lok Sibon Li,
Gerton Lunter, Sidney Markowitz, Vladimir Minin, Michael Defoin Platel,
Oliver Pybus, Tim Vaughan, Chieh-Hsi Wu, Walter Xie
Thanks to:
Roald Forsberg, Beth Shapiro and Korbinian Strimmer
Random number seed: 1751498377517
...
...
950000 -5968.4116 -5843.2135 -125.1981 0.3346 0.1999 0.2202 0.2451 7.5688 0.3837 8.8686 0.1018 0.3061 0.2627 0.2301 0.0990 1 1 1 0 1 1 0 1 1 0 0.1446 0.2821 0.1248 0.1256 0.0406 0.0402 0.1349 0.0389 0.0421 0.0257 0.2660 1m25s/Msamples
1000000 -5947.6786 -5828.5842 -119.0943 0.3356 0.2008 0.2158 0.2476 9.0574 0.2767 6.4146 0.1127 0.2849 0.1524 0.3138 0.1359 1 1 0 1 0 1 1 1 1 0 0.2870 0.2035 0.1539 0.0236 0.1138 0.0062 0.0249 0.0636 0.1174 0.0057 0.5793 1m25s/Msamples
Operator Tuning #accept #reject Pr(m) Pr(acc|m)
beast.base.inference.operator.BitFlipOperator(I.bitFlip) - 16124 36683 0.05277 0.30534
beast.base.inference.operator.kernel.BactrianDeltaExchangeOperator(R_trait.deltaExchange) 0.36270 6243 42499 0.04902 0.12808
kernel.BactrianScaleOperator(Theta.scale) 0.34135 3200 7426 0.01053 0.30115
kernel.BactrianScaleOperator(gamma.scale) 0.39153 3186 7382 0.01053 0.30148
kernel.BactrianScaleOperator(kappa.scale) 0.27344 2953 7609 0.01053 0.27959
kernel.BactrianScaleOperator(mu_trait.scale) 0.74609 3168 7339 0.01053 0.30151
beast.base.inference.operator.kernel.BactrianUpDownOperator(mu_traitUppsiDownOperator) 0.29436 112 146071 0.14650 0.00077 Try setting scale factor to about 0.147
beast.base.inference.operator.kernel.BactrianDeltaExchangeOperator(pi.deltaExchange) 0.03844 5260 17677 0.02272 0.22932
beast.base.inference.operator.kernel.BactrianDeltaExchangeOperator(pi_trait.deltaExchange) 0.25549 7346 20478 0.02779 0.26402
EpochFlexOperator(psi.BICEPSEpochAll) 0.05031 4892 12138 0.01711 0.28726
EpochFlexOperator(psi.BICEPSEpochTop) 0.05799 2496 8003 0.01053 0.23774
TreeStretchOperator(psi.BICEPSTreeFlex) 0.04849 45709 98680 0.14411 0.31657
Exchange(psi.narrowExchange) - 24785 119301 0.14411 0.17202
kernel.BactrianScaleOperator(psi.rootAgeScale) 0.22159 98 10445 0.01053 0.00930 Try setting scale factor to about 0.111
kernel.BactrianSubtreeSlide(psi.subtreeSlide) 0.48656 21723 122340 0.14411 0.15079
kernel.BactrianNodeOperator(psi.uniform) 1.78495 49685 94458 0.14411 0.34469
Exchange(psi.wideExchange) - 108 22148 0.02224 0.00485
WilsonBalding(psi.wilsonBalding) - 131 22105 0.02224 0.00589
Tuning: The value of the operator's tuning parameter, or '-' if the operator can't be optimized.
#accept: The total number of times a proposal by this operator has been accepted.
#reject: The total number of times a proposal by this operator has been rejected.
Pr(m): The probability this operator is chosen in a step of the MCMC (i.e. the normalized weight).
Pr(acc|m): The acceptance probability (#accept as a fraction of the total proposals for this operator).
Total calculation time: 87.253 seconds
End likelihood: -5947.678660781594
Done!
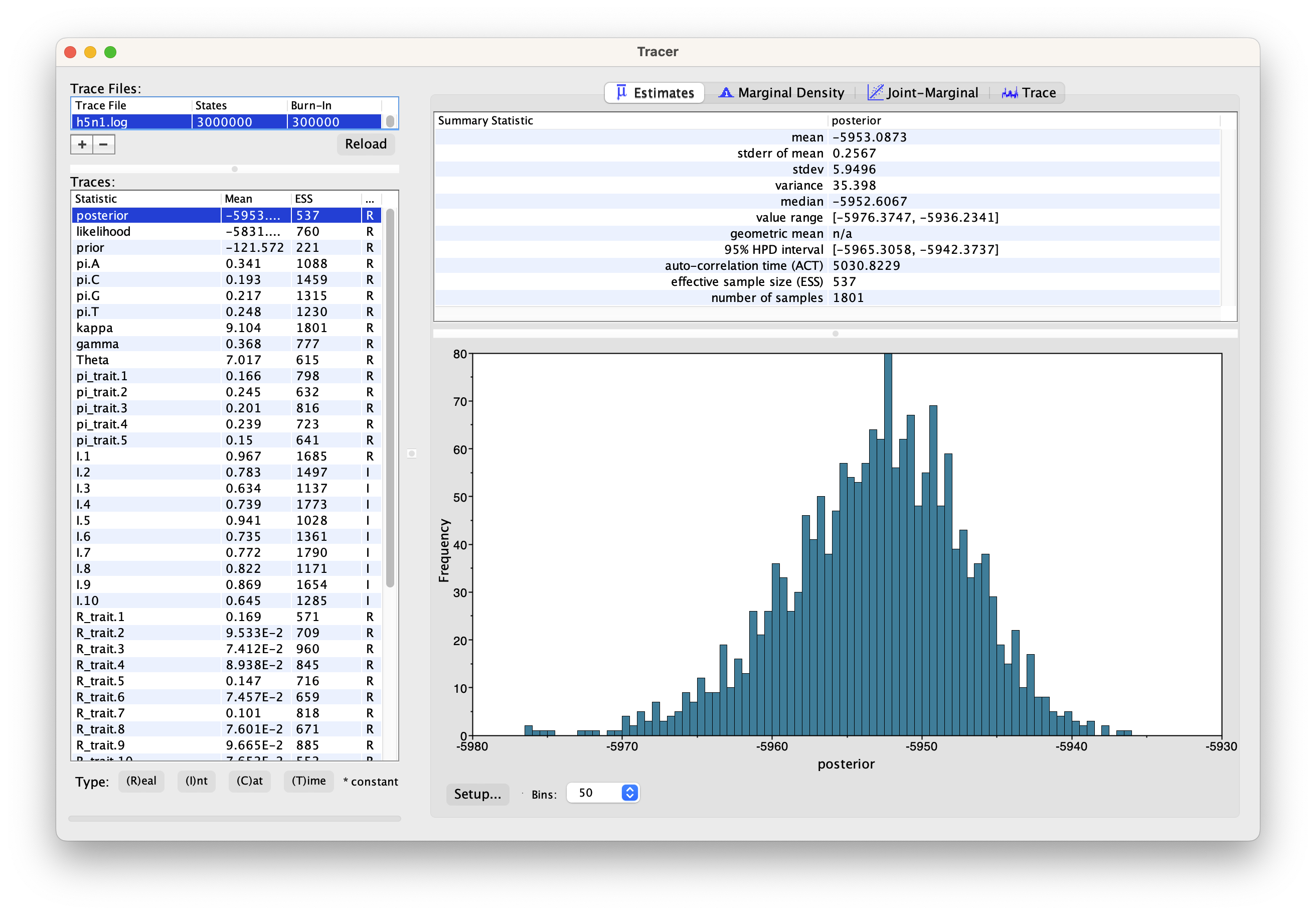
Analysing the BEAST output
Run the program called Tracer to analyze the output of BEAST. When the
main window has opened, choose Import Trace File... from the File menu
and select the file that BEAST has created called h5n1.log.
You should now see a window like in Figure 3.
Remember that MCMC is a stochastic algorithm so the actual numbers will
not be exactly the same.
On the left hand side is a list of the different quantities that BEAST has logged.
There are traces for the posterior (this is the log of the product of
the tree likelihood and the prior probabilities), and the continuous parameters.
Selecting a trace on the left brings up analyses for this trace on the right hand
side depending on tab that is selected. When first opened, the “posterior”” trace
is selected and various statistics of this trace are shown under the Estimates
tab. In the top right of the window is a table of calculated statistics for the
selected trace.
Tracer will plot a (marginal posterior) distribution for the selected parameter and also give you statistics such as the mean and median. The 95% HPD lower or upper stands for the highest posterior density interval and represents the most compact interval on the selected parameter that contains 95% of the posterior probability. It can be thought of as a Bayesian analog to a confidence interval.
In the implemented phylogeographic model, the rate matrix is symmetric, meaning that a relative migration rate between two locations solely represents a numerical value without implying any direction. Consequently, for 5 locations, there are only 10 relative migration rates, as illustrated in Figure 4.
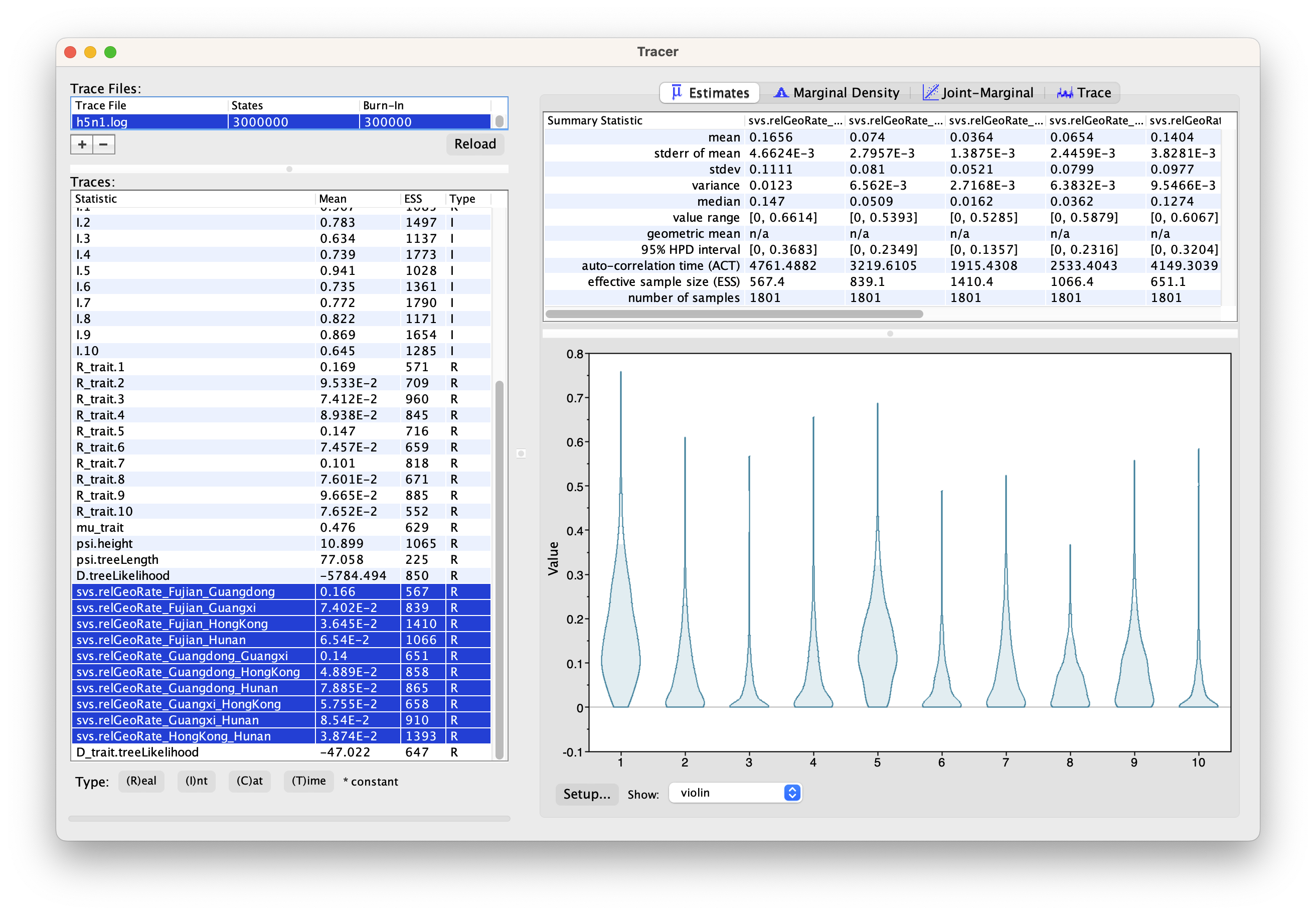
The h5n1.log is also provided as an additional resource, in case you need to use it for your analysis.
Summarizing posterior trees
To summarize the trees logged during MCMC, we will use the CCD methods implemented in the program TreeAnnotator to create the maximum a posteriori (MAP) tree. The implementation of the conditional clade distribution (CCD) offers different parameterizations, such as CCD1, which is based on clade split frequencies, and CCD0, which is based on clade frequencies. The MAP tree topology represents the tree topology with the highest posterior probability, averaged over all branch lengths and substitution parameter values.
You need to install CCD package to make the options available in TreeAnnotator.
Please follow these steps after launching TreeAnnotator:
- Set 10% as the burn-in percentage;
- Select “MAP (CCD0)” as the “Target tree type”;
- For “Node heights”, choose “Common Ancestor heights”;
- Load the tree log file that BEAST 2 generated (it will end in “.trees” by default) as “Input Tree File”. For this tutorial, the tree log file is called h5n1_with_trait.trees. If you select it from the file chooser, the parent path will automatically fill in and “YOUR_PATH” in the screen shot will be that parent path.
- Finally, for “Output File”, copy and paste the input file path but replace the h5n1_with_trait.trees with h5n1_with_trait.tree. This will create the file containing the resulting MAP tree.
The image below shows a screenshot of TreeAnnotator with the necessary settings to create the summary tree. “YOUR_PATH” will be replaced to the corresponding path.
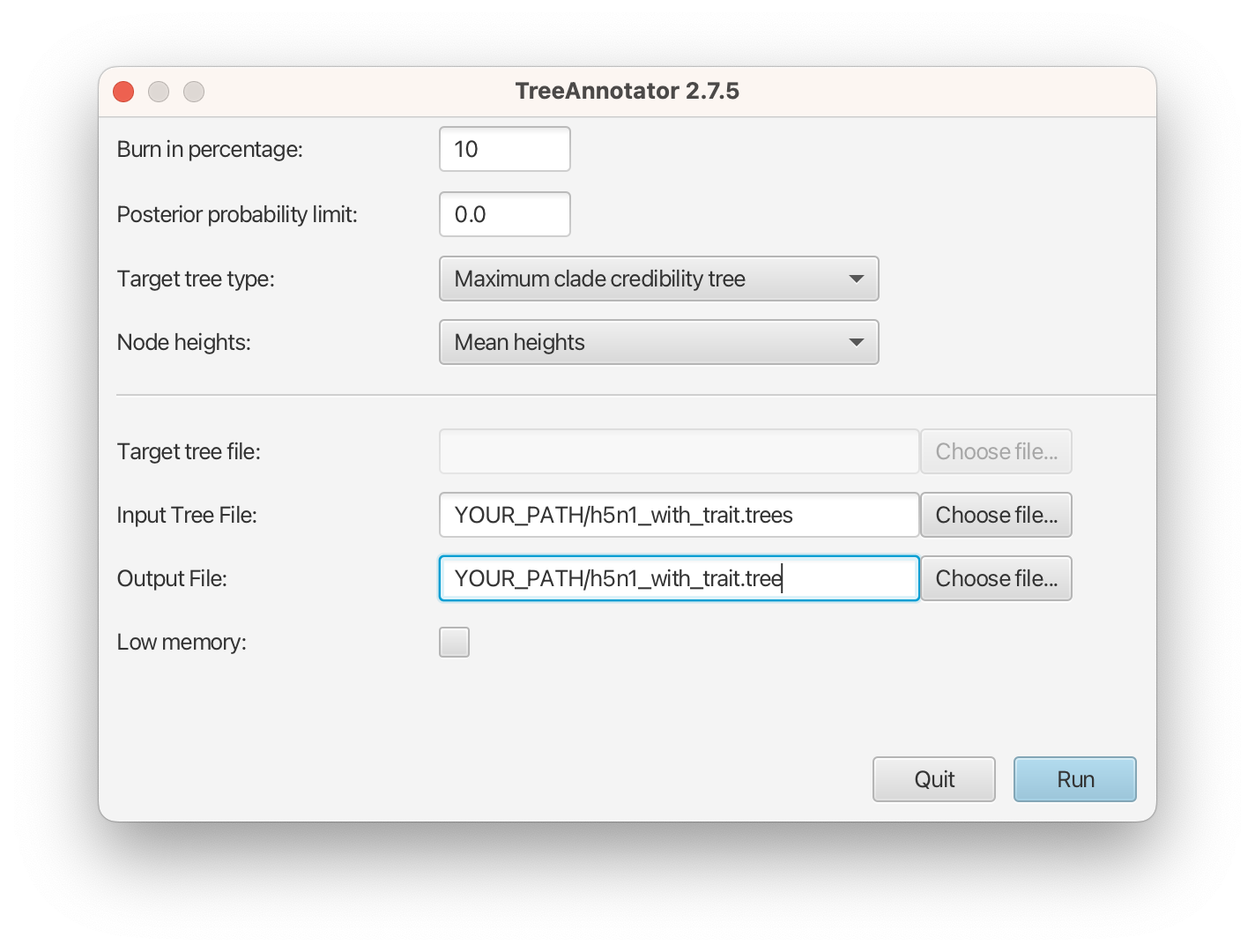
The setup described above will take the set of trees in the tree log file and summarize it with a single MAP tree using the CCD0 method. Divergence times will reflect the average ages of each node summarised by the selected method, and these times will be annotated with their 95% highest posterior density (HPD) intervals. TreeAnnotator will also display the posterior clade credibility of each node in the MAP tree. More details on summarizing trees can be found in beast2.org/summarizing-posterior-trees/.
Note that TreeAnnotator only parses a tree log file into an output text file, but the visualization has to be done with other programs (see next section).
Both h5n1_with_trait.trees and h5n1_with_trait.tree are provided as an additional resource, in case you need to use it for your analysis.
Distribution of root location
When you open the summary tree with locations h5n1_with_trait.tree in a text editor,
and scroll to the most right and locate the end of the tree definition,
you can see the set of meta data for the root.
Looking for the last entries of location.set and location.set.prob,
you might find something like this:
location.set = {Guangdong,HongKong,Hunan,Guangxi,Fujian}
location.set.prob = {0.1793448084397557,0.5985563575791227,0.03997779011660189,0.1265963353692393,0.0555247084952804}
This means that we have the following distribution for the root location:
| Location | Probability |
|---|---|
| Guangdong | 0.1793448084397557 |
| HongKong | 0.5985563575791227 |
| Hunan | 0.03997779011660189 |
| Guangxi | 0.1265963353692393 |
| Fujian | 0.0555247084952804 |
This distribution shows that the 95% HPD consists of all locations except Hunan, with a strong indication that HongKong might be the root with over 59% probability. It is quite typical that a lot of locations are part of the 95% HPD in discrete phylogeography.
Viewing the Location Tree
We can visualise the tree in a program called FigTree. There is a bug to launch the Mac version of FigTree v1.4.4. Feel free to visit our Tech Help page if you encounter any difficulties or need assistance.
Lauch the program, and follow the steps below:
- Open the summary tree file
h5n1_with_trait.treeby using theOpen...option in theFilemenu; - After the tree appears, expend
Appearanceand chooseColourbylocation; - Additionally, you can adjust the branch width based on the posterior support of
estimated locations by selecting
Width bylocation.prob. - Gradually increasing the
Line Weightcan make the branch widths vary more significantly based on their posterior supports. - To add a legend, tick
Legendand chooselocationfrom the drop-down list ofAttribute; - If you want to see the x-axis scale, you can tick
Scale Axis, expend it and uncheck the optionShow grid.
You should end up with something like Figure 6
Alternatively, you can use DensiTree to visulize the set of posterior trees
and set it up as follows:
- Use the
Loadoption in theFilemenu to load the trees fromh5n1_with_trait.trees(note this is NOT the summary tree, but the complete set); - Click
Showto chooseRoot Canaltree to guide the eye; - Click
Gridto chooseFull gridoption, type “2005” in theOrigintext field which is the year, and tickReverseto show the correct time scale; You also can reduce theDigitsto 0 which will rounding years in the x-axis (i.g. 2005, instead of 2005.22); - Go to
Line Color, you can colour branches bylocationand tickShow legend.
The final image look like Figure 7.
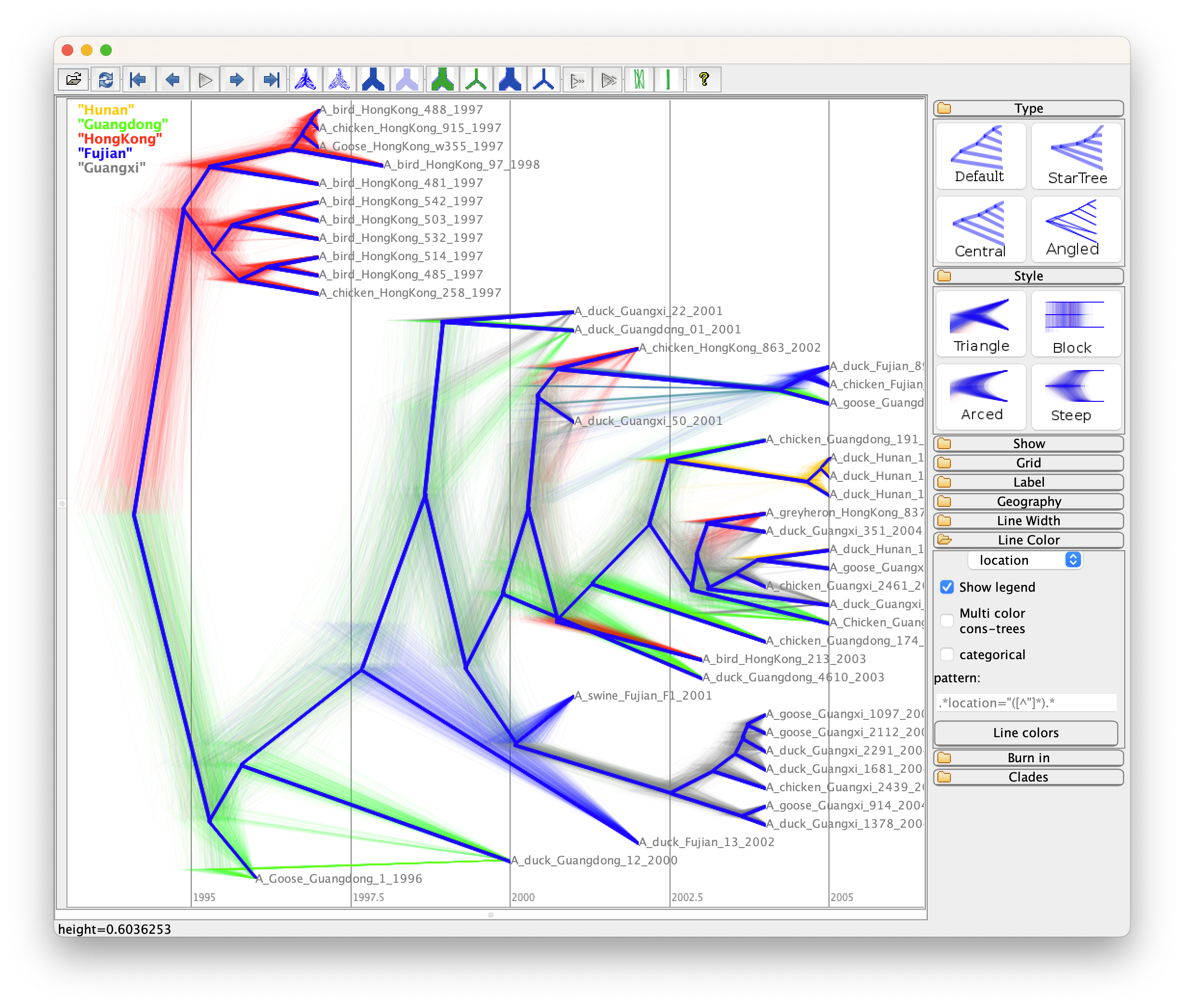
Bonus sections
The number of estimated transitions
To visualize how the location states change through the phylogeny,
you can use the StateTransitionCounter tool, which counts the number of branches
in a tree or a set of trees that have a certain state at the parent and another state at the node.
To use StateTransitionCounter, first, install the Babel package and then
run the tool through the BEAST application launcher.
The command below will generate the output file stc.out containing all counts
from the logged posterior trees h5n1_with_trait.trees, after removing 10% burn-in.
$BEAST2_PATH/bin/applauncher StateTransitionCounter -burnin 10 -in h5n1_with_trait.trees -tag location -out stc.out
Make sure you have installed the latest versions of Babel (>= v0.4.x) and BEASTLabs (>= v2.0.x) to ensure smooth execution.
Next, we will use R to plot the histogram based on the summary in stc.out.
If you encounter any issues generating it, you can download a prepared file
stc.out.
Additionally, download the script PlotTransitions.R,
which contains functions to parse the file and plot the histograms.
Before running the script, make sure you have installed the R packages ggplot2 and tidyverse.
Run the following scripts in R, where R_SRC_PATH is the folder containing R code,
and YOUR_WD is the folder containing the stc.out file:
setwd(R_SRC_PATH)
source("PlotTransitions.R")
setwd(YOUR_WD)
# stc.out must be in YOUR_WD
stc <- parseTransCount(input="stc.out", pattern = "Histogram", target="Hunan")
# only => Hunan
p <- plotTransCount(stc$hist[grepl("=>Hunan", stc$hist[["Transition"]]),],
colours = c("Fujian=>Hunan" = "#D62728", "Guangdong=>Hunan" = "#C4C223",
"Guangxi=>Hunan" = "#60BD68", "HongKong=>Hunan" = "#1F78B4"))
ggsave( paste0("transition-distribution-hunan.png"), p, width = 6, height = 4)
The stc file contains a statistical summary of the estimated transition counts
related to the target location, which is Hunan, and a table of the actual counts.
To plot a simple graph, we will pick up the transitions to Hunan in the next command
and then save the graph as a PNG file. The counts will be normalized into probabilities.
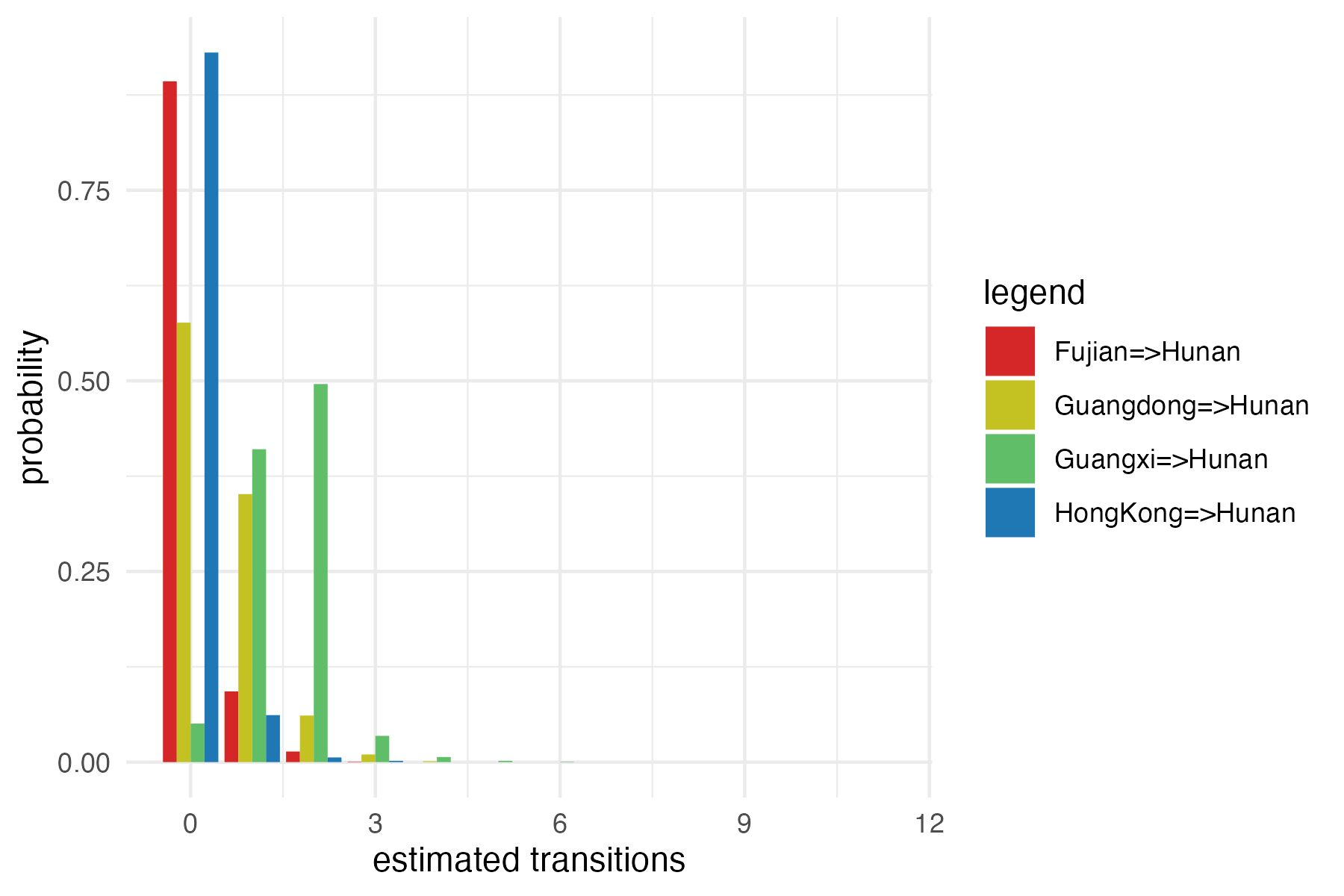
The x-axis represents the number of estimated transitions in all migration events from one particular location to another, separated by “=>”, and the y-axis represents the probability, which is normalized from the total counts. From the graph, we can observe that “Guangxi => Hunan” (in blue) has a higher probability than other migration events. This type of visualization quantifies the uncertainty in how the disease (H5N1) spreads between locations of interest, as simulated from your model and given the data. For more details and visualizations, you can refer to the work of Douglas et. al. 2020.
Visualizing phylogeographic diffusion
To analyze and visualize phylogeographic reconstructions resulting from Bayesian inference of
spatio-temporal diffusion based on the Bielejec et al., 2011 method,
we can use a software called spread.
Please note it requires Java 1.8. If you run into any issues or need support, don’t hesitate to check out our Tech Help page.
We recommend to download the .jar file from the Spread website, and follow the steps below:
- Start the
spreadapplication by using the commandjava -jar SPREADv1.0.7.jar. - Select the
Openbutton in the panel underLoad tree file. - Choose the summary tree file
h5n1_with_trait.tree`. - Change the
State attribute nameto the name of the trait, which islocationin this analysis. - Click the
Setupbutton to edit altitude and longitude for the locations. You can also load this information from a tab-delimited file, and a prepared file locationCoordinates_H5N1.txt is also available. Remeber to clickDonebutton to save the information into spread. - Change the
Most recent sampling dateto2005. - Open the
Outputtab in the left-hand side panel, and then choose where to save the KML file (default isoutput.kml). - Click the
Generatebutton to create the KML file.
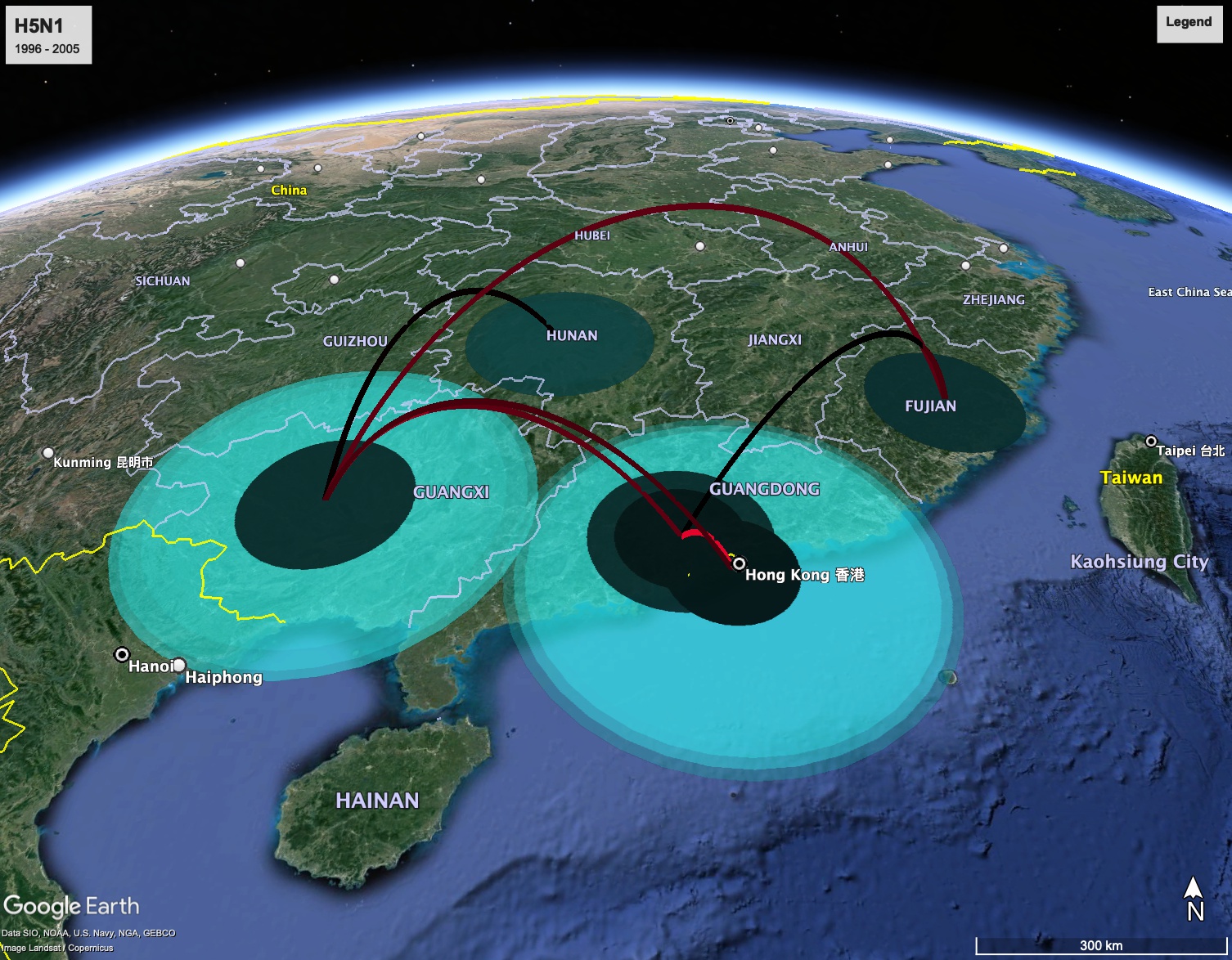
The world map with the tree superimposed onto the area where the rabies epidemic occurred will be displayed. If you encounter any issues generating the KML file, you can download a prepared output.kml.
The KML file can be imported into Google Earth, allowing you to animate the spread of the epidemic through time. The colored areas on the map represent the 95% Highest Posterior Density (HPD) regions of the locations of the internal nodes of the summary tree.
Programs used in this tutorial
The following software will be used in this tutorial. We also offer a Tech Help page to assist you in case you encounter any unexpected issues with certain third-party software tools.
-
Java 17 or a later version is required by LPhy Studio.
-
LPhy Studio - This software will specify, visualize, and simulate data from models defined in LPhy scripts. At the time of writing, the current version is v1.7.x. It is available for download from LPhy releases.
-
LPhy BEAST - this BEAST 2 package will convert LPhy scripts into BEAST 2 XMLs. The installation guide and usage can be found from User Manual.
-
BEAST 2 - the bundle includes the BEAST 2 program, BEAUti, DensiTree, TreeAnnotator, and other utility programs. This tutorial is written for BEAST v2.7.x or higher version. It is available for download from http://www.beast2.org. Use
Package Managerto install the required BEAST 2 packages. -
BEAST labs package - this contains some generally useful stuff used by other BEAST 2 packages.
-
BEAST feast package - this is a small BEAST 2 package which contains additions to the core functionality.
-
BEAST CCD package - Implementation of the conditional clade distribution (CCD), such as, based on clade split frequencies (CCD1) and clade frequencies (CCD0). Furthermore, point estimators based on the CCDs are implemented, which allows TreeAnnotator to produce better summary trees than via MCC trees (which is restricted to the sample).
-
Tracer - this program is used to explore the output of BEAST (and other Bayesian MCMC programs). It graphically and quantitatively summarises the distributions of continuous parameters and provides diagnostic information. At the time of writing, the current version is v1.7.x. It is available for download from Tracer releases.
-
FigTree - this is an application for displaying and printing molecular phylogenies, in particular those obtained using BEAST. At the time of writing, the current version is v1.4.x. It is available for download from FigTree releases.
-
BEAST classic package - a BEAST 2 package includes the discrete phylogeography model introduced in this tutorial.
-
Babel - a BEAST 2 package contains tools for post-analysis. We will use
StateTransitionCounterto estimate the number of transitions among locations. -
Spread - it summarises the geographic spread and produces a KML file, which is available from https://rega.kuleuven.be/cev/ecv/software/spread.
-
Spread requires Java 1.8.
-
Google earth - it is used to display the KML file (just Google for it, if you have not already have it installed).
Data
The number of replicates, L is the number of characters of alignment, D. The alignment, D is read from the Nexus file with a file name of "data/H5N1.nex" and options. The options are ageDirection="forward" and ageRegex="_(\d+)$". The taxa is the list of taxa of alignment, D. The int, K is the number of states of alignment, Dtrait. The alignment, Dtrait extracts the trait from taxa, with a sep of "_" and an i of 2. The object, dim comes from the K*(K-1)/2. The data type used for simulations, dataType is the data type of alignment, Dtrait.Model
The alignment, D is assumed to have evolved under a phylogenetic continuous time Markov process (Felsenstein; 1981) on phylogenetic time tree, ψ, with a molecular clock rate of 0.004, an instantaneous rate matrix and siteRates, r. An instantaneous rate matrix is the HKY model (Hasegawa et al; 1985) with transition bias parameter, κ and base frequency vector, π. The base frequency vector, π have a Dirichlet distribution prior with a concentration of [2.0, 2.0, 2.0, 2.0]. The transition bias parameter, κ has a log-normal prior with a mean in log space of 1.0 and a standard deviation in log space of 1.25. The double, ri is assumed to come from a DiscretizeGamma with shape, γ and a ncat of 4, for i in 0 to L - 1. The shape, γ has a log-normal prior with a mean in log space of 0.0 and a standard deviation in log space of 2.0. The time tree, ψ is assumed to come from a Kingman's coalescent tree prior (Kingman; 1982) with coalescent parameter, Θ and taxa. The coalescent parameter, Θ has a log-normal prior with a mean in log space of 0.0 and a standard deviation in log space of 1.0. The alignment, Dtrait is assumed to have evolved under a phylogenetic continuous time Markov process (Felsenstein; 1981) on phylogenetic time tree, ψ, with molecular clock rate, μtrait, instantaneous rate matrix, Qtrait, a length of 1 and a dataType of "standard". The instantaneous rate matrix, Qtrait is assumed to come from the generalTimeReversible with rates and freq, πtrait. The freq, πtrait have a Dirichlet distribution prior with a concentration. A concentration is assumed to come from the rep with an element of 3.0 and times, K. The number is assumed to come from the select with x, R_traiti and indicator, I0. The indicator, I is assumed to come from a Bernoulli with a p of 0.5, replicates, dim and minSuccesses. The minSuccesses is calculated by dim-2. The x, Rtrait have a Dirichlet distribution prior with a concentration. A concentration is assumed to come from the rep with an element of 1.0 and times, dim. The molecular clock rate, μtrait has a log-normal prior with a mean in log space of 0 and a standard deviation in log space of 1.25.Posterior
$$ \begin{split} P(\boldsymbol{\pi}, \kappa, \boldsymbol{r}, \gamma, \boldsymbol{\psi}, \Theta, \boldsymbol{\pi\textbf{}_{trait}}, \boldsymbol{I}, \boldsymbol{\textbf{R}_{trait}}, \mu\textrm{}_{trait} | \boldsymbol{D}, \boldsymbol{\textbf{D}_{trait}}) \propto &P(\boldsymbol{D} | \boldsymbol{\psi}, Q, \boldsymbol{r})P(\boldsymbol{\pi})P(\kappa)\\& \prod_{i=0}^{L - 1}P(\textrm{r}_i | \gamma)P(\gamma)P(\boldsymbol{\psi} | \Theta)\\& P(\Theta)P(\boldsymbol{\textbf{D}_{trait}} | \boldsymbol{\psi}, \mu\textrm{}_{trait}, \boldsymbol{\textbf{Q}_{trait}})\\& P(\boldsymbol{\pi\textbf{}_{trait}})P(\boldsymbol{I})P(\boldsymbol{\textbf{R}_{trait}})\\& P(\mu\textrm{}_{trait})\end{split} $$Useful Links
- LinguaPhylo: https://linguaphylo.github.io
- BEAST 2 website and documentation: http://www.beast2.org/
- Join the BEAST user discussion: http://groups.google.com/group/beast-users
References
- Felsenstein, J. (1981). Evolutionary trees from DNA sequences: a maximum likelihood approach. Journal of molecular evolution, 17(6), 368-376. https://doi.org/10.1007/BF01734359
- Hasegawa, M., Kishino, H. & Yano, T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol 22, 160–174 (1985) https://doi.org/10.1007/BF02101694
- Kingman JFC. The Coalescent. Stochastic Processes and their Applications 13, 235–248 (1982) https://doi.org/10.1016/0304-4149(82)90011-4
- Lemey, P., Rambaut, A., Drummond, A. J. and Suchard, M. A. (2009). Bayesian phylogeography finds its roots. PLoS Comput Biol 5, e1000520.
- Wallace, R., HoDac, H., Lathrop, R. and Fitch, W. (2007). A statistical phylogeography of influenza A H5N1. Proceedings of the National Academy of Sciences 104, 4473.
- Bielejec F., Rambaut A., Suchard M.A & Lemey P. (2011). SPREAD: Spatial Phylogenetic Reconstruction of Evolutionary Dynamics. Bioinformatics, 27(20):2910-2912. doi:10.1093.
- Douglas, J., Mendes, F. K., Bouckaert, R., Xie, D., Jimenez-Silva, C. L., Swanepoel, C., … & Drummond, A. J. (2020). Phylodynamics reveals the role of human travel and contact tracing in controlling COVID-19 in four island nations. doi: https://doi.org/10.1101/2020.08.04.20168518